The FAT and the LIPID: Triglyceride that thickens your hide.
Le Intro,
Lipids are one of the major constituents of foods, and are important in our diet, especially the cholesterol. Yes, cholesterol is the one who actually make the hormones inside our body. (they the main components that make them, sort of) Well, that is some of the function of lipids. Other and Main Function of the lipid is to provide energy, give essential nutrients and prevent cold temperature's by becoming body's heat insulator. (feel that flabby in your tummy? that's lipid)
Most people actually use the term fat for lipid. They are actually the same thing, but in academic world, we prefer to call lipid, since the word fat can become derogatory for some.... (don't quote on me for this)
Most people also tend to think that cholesterol is the lipid, but in actuality, cholesterol is just a subset of lipid. Yeah, there's more than cholesterol in lipid. There's Triglyceride a.k.a trigacylglyceride (TAG @ TCG), phospholipids and sterols. I'll cover most of them later, but in the mean time, I'll talk about TAG.
 |
| Triglyceride structure. Notice the colour? the red is fatty acid, and the green is a glycerol. |
AS according to the picture, the red is a fatty acid and the green is a glycerol.
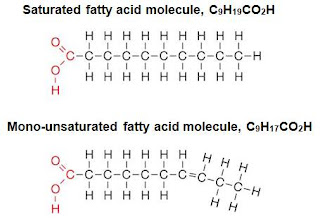
The fatty acid is a carbon chain skeleton that contain the element Carbon (C), Hydrogen (H), and Oxygen (O) and it is arranged with a carbonyl group at 1 end (-COOH). As in the picture, the carbonyl group is not to be seen as it was used to link the fatty acid with the glycerol. But that's not all, the bond that "stick" it all together is also important. There's Saturated Fatty Acids (SFA's) where the carbon can hold all the hydrogen; consists of only single bond and no double bond. Thus, the term saturated used. Monounsaturated Fatty Acids (MUFA's) have only one double bond in it and Polyunsaturated Fatty Acid's (PUFA's) have several double bond. As a refresher for all those who take organic chemistry, SFA's are Alkanes (Cn H2n+2), MUFA's and PUFA's are alkenes (Cn H2n). There are A LOT of Fatty acid in the world, some that is well known is Palmitic Acid (C16 H34), Oleic Acid (C18 H36) and Linoleic Acid (C18 H34). Try to know why these fatty acid is famous in your free time, ok?
So, what's this story gotta do with the Triglyceride? So here it is, the one that bind the fatty acids together is a Glycerol. But first, what is this Glycerol?
Well, Glycerol is a Tryhidric Alcohol (3 -OH Hydroxyl Group); where each hydroxyl Group were able to bind with a carbonyl Group, hence the fatty-acid chain formed. Diverse type of fatty acid combination attached to the glycerol leading to various type of TAG. And sometimes, not all hydroxyl group of the glycerol managed to bond with something, so there are Diglyceride and Monoglyceride. Not to mention, the glycerol can bond to any fatty acid; meaning, the fatty acid in the TAG doesn't necessary bond to the same fatty acid for all 3 hydroxyl group. The TAG, may bind with a palmitic acid in 1 chain, Oleic acid in 1 chain and Linoleic acid n the other. (Well, there are several conditions on that for it to happened, such as the hydrogen bonding effect and all that's other bonding stuff, but this is no textbook, it's only a blog).
So, there you have it, Some lipid it was, and this was just the Triglyceride! There's still phospholipid, Sterol and Cholesterol! Oh, this is not done yet.
But for today, it is done.
Thank you for reading, hope this shed some light.
No comments:
Post a Comment